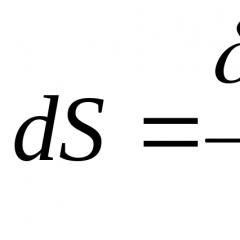A sign of which reaction is the release of gas. Signs of chemical reactions occurring. Ionic reactions occur between ions that are already present or formed during the reaction
From the previous sections we learned (approximately, of course) what substances there are and how they are structured. Now we need to get acquainted with the most important thing in chemistry - chemical reactions: find out what they are, why some substances react and others do not, and why reactions go this way and not otherwise. When chemistry appeared as a science (and this happened approximately in the 17th - 18th centuries), chemists dealt with a small number of known elements and with a relatively small number of substances.
However, they had very little idea of what happens during a chemical reaction when one substance is converted into another. Chemistry in those days was a set of empirical rules, that is, rules found as a result of numerous experiments, often carried out without any pre-planned plan.
And chaos often reigned in the heads of chemists - just like many schoolchildren now! Prominent American physical chemist George Hammon spoke about this: “In the 1950s, organic chemistry textbooks became so large that they were divided into two parts.
And you had to remember every connection, every reaction. And the best students learned all this.
It was painful, but it was required - to remember the names of all these compounds, all these reactions...
» In fact, order reigns in modern chemistry; chemists know that it has already been precisely established what requires verification, and what is still unknown to them. Here is the main thing that was established long ago and precisely: in chemistry the law of conservation of the number of atoms is strictly observed.
In chemical processes, some elements cannot be converted into others, and any chemical reaction is simply a “rearrangement of atoms”: the atoms that were part of the starting substances (often called reactants) end up in the reaction products. In this case, the number of atoms of each element remains strictly constant.
A modern chemist will never try to carry out “impossible” transformations, for example, to obtain gold from mercury or lead, as alchemists tried to do. Or obtain fluorine oxide F2O7, in which this element would be heptavalent, despite the fact that the valence shell of its atom contains seven electrons, and in this respect fluorine is similar to chlorine, the oxide of which C12O7 is known.
And only as a joke, a chemist can write “equations” of such reactions as A1 + Cu = Au + C1 or Si + Nb = Sb + Ni (try it yourself, using periodic table, compose several more such “alchemical transformations”). At all times, and now too, main question for chemists - how to obtain a substance with the desired properties.
But before answering it, it is necessary to find out what will happen if such and such substances react.
And it would also be good to know in advance at what speed a specific reaction will take place under given conditions.
Too slow is bad, wait a long time, but too fast can also be bad: as if there was no explosion... It is known that many substances can calmly coexist without reacting with each other at all.
Beginners studying chemistry sometimes ask a question that confuses the teacher: what happens if you take a little out of all these jars of reagents and mix everything? But even if such a strange experiment is carried out, the following question will immediately arise: how to find out whether a chemical reaction occurred when mixing certain substances or whether there was no reaction at all?
Chemists have long isolated characteristic features chemical reaction. It is usually believed that the course of a reaction is characterized by the release of heat (and sometimes light, as well as sound), the formation of a precipitate, and the release of gaseous substances.
Here specific examples. If you pour a heap of powdered ammonium dichromate onto an iron sheet and set it on fire, a very beautiful reaction is observed: (NH4)2Cr2O7 = Cr2O3 + N2 + 4H2O.
At the same time, sparks fly upward from the red hill, and green chromium oxide powder is released in all directions, like lava.
No wonder this experiment was called “Volcanic Eruption.” In this reaction, light, heat, and gases (nitrogen and water vapor) are released.
These are all characteristic signs of a chemical reaction.
Everyone knows that heat and light accompany combustion reactions.
But there are exceptions here too.
For example, if you ignite a stream of hydrogen, its flame will be completely invisible. True, for this, hydrogen must be released from the metal tube, since the glass one will quickly heat up at the end and color the flame yellow (sodium glow).
To make sure that the hydrogen coming out of the tube actually burns, a cold object is brought to its outlet, and then droplets of water formed in the combustion reaction are deposited on it: 2H2 + O2 = 2H2O.
Reactions with the release of light, but without combustion, are also known. This phenomenon is called chemiluminescence.
Rotflies, fireflies, and some marine unicellular organisms can glow. Many marine animals living both on the surface of the sea and in its depths also glow.
These are examples of bioluminescence - glow in living organisms. In all these cases, the energy of the chemical reaction is released in the form of light.
In 1669, the Hamburg alchemist Hennig Brand accidentally discovered white phosphorus by its glow in the dark. Subsequently, chemists found that white phosphorus easily evaporates and its vapors glow when they react with oxygen in the air.
Light is also released in the reaction of some organic substances with hydrogen peroxide. In this case, such bright chemiluminescence is observed that it can be seen even in daylight.
This phenomenon is used, for example, to produce toys and jewelry. They are made in the form of transparent plastic tubes in which an ampoule with hydrogen peroxide is sealed, as well as a solution of a complex substance - oxalic acid diphenyl ester and a fluorescent dye.
If the ampoule is crushed, the ether will begin to oxidize, the energy of this reaction is transferred to the dye, which glows. Its color can be different - orange, blue, green - depending on the dye.
The faster the oxidation reaction occurs, the brighter the glow, but the faster it stops.
By selecting the components, a bright (can be read in the dark) glow is obtained, which fades within about 12 hours - this is quite enough for a carnival or disco. Here are examples of reactions accompanied by the release of a large amount of heat.
If you pour calcium oxide powder (quicklime) with water, the reaction results in the formation of slaked lime (calcium hydroxide): CaO + H2O = Ca(OH)2. This reaction releases so much heat that water boils in a glass placed in quicklime before the experiment. Another example is taken from the biography of the American physicist Robert Wood.
Once he took his bride for a sleigh ride, and her hands were frozen.
Then Wood took out a bottle he had stored, three-quarters filled with water, and poured concentrated sulfuric acid into it from the bottle. “After ten seconds, the bottle became so hot,” the future famous physicist wrote in his diary, “that it could not be held in your hands.
When it began to cool, I added more acid, and when the acid stopped raising the temperature, I took out a jar of caustic soda sticks and added them little by little. In this way the bottle was heated almost to a boil the entire trip.”
The reaction of sulfuric acid with sodium hydroxide (the old name for sodium hydroxide) goes like this: H2SO4 + 2NaOH = Na2SO4 + 2H2O.
This reaction actually releases a lot of heat.
What reaction occurs when sulfuric acid is simply diluted? There has been a dispute about this for a long time.
Many chemists believed that there was no chemical reaction in this case. Others, including D.I. Mendeleev, believed that there was still a chemical reaction sulfuric acid with water. Nowadays, such processes are considered to be physicochemical.
Chemical reactions are detected by various accompanying phenomena.
Heating a mixture of substances, glowing, light flashes, explosions. It is not difficult to understand that all this is the result of the release of energy. Chemical reactions should be carried out with great care, protecting primarily the eyes, keeping vessels with substances as far away from the face as possible. If the result of a reaction is not known in advance, then experiments are carried out with very small quantities of substances. Work with volatile, toxic, or strong-smelling substances cannot be carried out in enclosed spaces without good exhaust ventilation (draft).
EXPERIENCE 2.1. A little bromine Br 2 (a heavy red-brown liquid with a pungent odor) and aluminum shavings are placed in a thick-walled test tube (Fig. 2.1). The test tube is closed with a stopper with a long tube. The reaction is slow at first and the changes are not noticeable. It gradually speeds up and ends with a bright flash. The mixture heats up, and the vapor of unreacted excess bromine rises high up the tube. Reaction equation:
Change in color. It is quite natural that the starting substances and reaction products are characterized by different properties, including that they may have completely different colors. Color changes can be demonstrated by many interesting experiments.
EXPERIENCE 2.2. Add 3-5 drops of copper sulfate solution CuS0 4 to a test tube with a dilute ammonia solution. An intense purple color appears due to the formation of a new substance:
Gas release. Gaseous substances as reaction products can be released from solutions, as well as from molten and solid mixtures. Many gas bubbles rise to the surface of the liquid. Liquid splashing occurs, which should be avoided. Sometimes foam forms. When performing such experiments, the vessels should not be tightly closed.
EXPERIENCE 2.3. Crystalline sodium nitrate NaNOg is placed in a test tube with a layer of about 5 mm. Heat carefully in the flame of a gas burner until melting (308 ° C). The release of gas bubbles begins, in which the smoldering splinter lights up. This proves that the gas released is oxygen. More than 60 cm 3 of oxygen is released from 0.5 g of NaN0 3:

Rice. 2.1. Device for carrying out the reaction of aluminum with bromine

Rice. 2.2. Device for producing gas
To collect gas in the form of an individual substance, use various devices, one of which is shown in Fig. 2.2. The formation or absorption of gaseous substances is associated with the appearance and disappearance of odor. This is also a practically important sign of the occurrence of chemical reactions.
experience 2 .4. In a porcelain mortar, grind calcium hydroxide powder Ca(OH) 2 with ammonium chloride NH 4 C1. An ammonia smell appears:
Formation of precipitation. Chemical reactions in solutions often lead to the formation of substances that are insoluble in water or other liquids. Reactions in aqueous solutions are usually considered.
experience 2.5. A yellow solution of potassium chromate K 2 Cr0 4 is added to a solution of lead nitrate Pb(N0 3) 2. An insoluble bright yellow substance, lead chromate PbCr04, appears and settles to the bottom of the vessel. This substance is used as a pigment - crown yellow.
task 2 .1. What conditions facilitate the occurrence of chemical reactions? To answer, use the data from the examples given, as well as your observations and assumptions.
task 2.2. A solution of potassium chromate was added to two test tubes containing a solution of lead nitrate without measuring the volume. After lead chromate settled to the bottom, the solution in one of the test tubes turned out to be yellow and in the other colorless. What will be observed when potassium chromate solution is added to the test tubes again?
Classification of chemical reactions
Let's consider the classification of chemical reactions according to changes in the number and composition of the starting substances and reaction products.
Compound reactions. One product can be formed from two starting substances. This type of transformation is called connection reaction.
When sufficiently heated on an iron plate, a mixture of gray zinc powder and yellow sulfur powder begins to heat up to a light red glow. Some of the sulfur evaporates. After the reaction is completed, the product cools and turns into a white mass of zinc sulfide ZnS:
![]()
Reactions of this type are possible both between simple and between complex substances. White calcium oxide powder, or quicklime CaO, when mixed with water, heats up, turning into a loose white mass - calcium hydroxide Ca(OH) 2, or slaked lime:
Decomposition reactions. When conditions change, a substance can transform into two or more new substances. The corresponding reactions are called decomposition reactions.
Blue powder of copper hydroxide Cu(OH) 2 or a precipitate of this substance in a test tube with a solution when slightly heated (70-90 ° C) turns black, turning into copper oxide CuO:
There are also very unstable substances that decompose with the slightest heating, and also exist only at low temperatures.
Lead (IV) chloride PbC1 4 at room temperature is a yellow liquid. When heated slightly, it decomposes explosively, turning into lead chloride (P):
Substitution reactions. Atoms or groups of atoms that make up one of the reactants can replace some of the atoms in another reactant. This interaction of substances is called substitution reaction.
experience 2.6. Iron in the form of sawdust or a small product (nail, paper clip), dipped in hydrochloric acid (a solution of hydrogen chloride HC1 in water), replaces hydrogen, forming a pale green solution of iron(I) chloride:
The hydrogen released can be collected using the device shown in Fig. 2.2, but replacing the large flask with a test tube. When a lit match approaches the test tube, the hydrogen instantly burns and a characteristic whistling sound is heard.
When a mixture of Na 2 C0 3 soda with white quartz sand Si0 2 is strongly heated, the CO 2 group released in the form of carbon dioxide is replaced by the Si0 2 group:
After calcination, white sodium silicate remains.
Exchange reactions. Reactants can exchange atoms or groups of atoms, and this interaction is called exchange reaction.
The separation of a precipitate from a solution very often occurs as a result of exchange. When colorless solutions of barium chloride BaCl 2 and magnesium sulfate MgS0 4 are mixed, a white suspension (suspension) of water-insoluble barium sulfate BaS0 4 is formed, which
gradually settles to the bottom of the test tube. Above the precipitate is a colorless solution of magnesium chloride:
The reaction of barium sulfate formation is often used to analyze (test) solutions for the presence of compounds of the chemical element barium.
exercise 2 .3. Determine what type of reaction between lead nitrate and potassium chromate is (p. 45).
exercise 2.4. Find other examples of exchange reactions in the material you have studied.
Transfer reactions. There are chemical reactions characterized by the fact that an atom or group of atoms moves from a structural unit of one substance to a structural unit of another substance. They are called transfer reactions.
EXPERIENCE 2.7. A colorless solution of tin(II) chloride SnCl 2 is added to the water-insoluble white powder of silver chloride AgCl. The mixture turns black due to the formation of small grains of silver. Chlorine atoms move from silver chloride to tin chloride:
The transfer reaction can proceed as a real transport of particles from one substance to another. If in a special vessel desiccator(Fig. 2.3) place blue crystals in open cups copper sulfate CuS0 4 5H 2 0 and white phosphorus oxide powder P 2 0 5, then after a few days the crystals turn white, losing water, and the phosphorus oxide

Rice. 2.3. Desiccator with substances involved in water transfer
pa reacts with it, turning into metaphosphoric acid:
Water is transported in the form of steam through airspace in a desiccator.
QUESTIONS AND EXERCISES
1. Give your own examples of reactions accompanied by characteristic phenomena.
2. What types of reactions are considered in section 2.1?
3. Ammonium carbonate (NH 4) 2 C0 3, white powder, has a faint odor of ammonia. In the open air, the substance gradually disappears, decomposing into gaseous substances. Write the reaction equation.
4. What type of reactions are the following:

Lesson type: acquisition of new knowledge.
Lesson type: conversation with demonstration of experiments.
Goals:
Educational- repeat the differences between chemical phenomena and physical ones. To develop knowledge about the signs and conditions of chemical reactions.
Developmental- develop skills, based on knowledge of chemistry, pose simple problems, formulate hypotheses, generalize.
Educational – continue the formation of the scientific worldview of students, cultivate a culture of communication through work in pairs “student-student”, “student-teacher”, as well as observation, attention, inquisitiveness, and initiative.
Methods and methodological techniques: Conversation, demonstration of experiments; filling out the table, chemical dictation, independent work with cards.
Equipment and reagents. Laboratory stand with test tubes, an iron spoon for burning substances, a test tube with a gas outlet tube, an alcohol lamp, matches, solutions of iron chloride FeCL 3, potassium thiocyanate KNCS, copper sulfate (copper sulfate) CuSO 4, sodium hydroxide NaOH, sodium carbonate Na 2 CO 3, hydrochloric acid HCL, powder S.
Lesson progress
Teacher. We are studying the chapter “Changes that occur in substances” and we know that changes can be physical and chemical. What is the difference between a chemical phenomenon and a physical one?
Student. As a result of a chemical phenomenon, the composition of a substance changes, and as a result of a physical phenomenon, the composition of a substance remains unchanged, and only its state of aggregation or the shape and size of bodies changes.
Teacher. Chemical and physical phenomena can be observed simultaneously in the same experiment. If you flatten copper wire with a hammer, you get a copper plate. The shape of the wire changes, but its composition remains the same. This physical phenomenon. If a copper plate is heated over high heat, the metallic luster will disappear. The surface of the copper plate will be covered with a black coating, which can be scraped off with a knife. This means that copper interacts with air and turns into a new substance. This is a chemical phenomenon. A chemical reaction occurs between the metal and oxygen in the air.
Chemical dictation
Option 1
Exercise. Indicate what phenomena (physical or chemical) you are talking about. Explain your answer.
1. Combustion of gasoline in a car engine.
2. Preparation of powder from a piece of chalk.
3. Rotting of plant residues.
4. Souring of milk.
5. Rainfall
Option 2
1. Coal burning.
2. Melting snow.
3. Formation of rust.
4. Frost formation on trees.
5. Glow of a tungsten filament in a light bulb.
Evaluation criteria
You can score a maximum of 10 points (1 point for correctly indicated phenomenon and 1 point for justifying the answer).
Teacher. So, you know that all phenomena are divided into physical and chemical. Unlike physical phenomena, during chemical phenomena, or chemical reactions, the transformation of some substances into others occurs. These transformations are accompanied by external signs. In order to introduce you to chemical reactions, I will conduct a series of demonstration experiments. You need to identify signs that indicate a chemical reaction has occurred. Pay attention to what conditions are necessary for these chemical reactions to occur.
Demonstration experience No. 1
Teacher. In the first experiment, you need to find out what happens to ferric chloride (111) when a solution of potassium thiocyanate KNCS is added to it.
FeCL 3 + KNCS = Fe(NCS) 3 +3 KCL
Student. The reaction is accompanied by a color change
Demonstration experiment No. 2
Teacher. Pour 2 ml of copper sulfate into a test tube and add a little sodium hydroxide solution.
CuSO 4 + 2 NaOH = Cu (OH) 2↓ + Na 2 SO 4
Student. A blue precipitate appears, Cu(OH) 2↓
Demonstration experiment No. 3
Teacher. To the resulting solution of Cu (OH) 2↓ add a solution of acid HCL
Cu (OH) 2↓ + 2 HCL = CuCL 2 +2 HOH
Student. The precipitate dissolves.
Demonstration experiment No. 4
Teacher. Pour a solution of hydrochloric acid HCL into a test tube containing a solution of sodium carbonate.
Na 2 CO 3 +2 HCL = 2 NaCL + H 2 O + CO 2
Student. Gas is released.
Demonstration experiment No. 5
Teacher. Let's set a little sulfur on fire in an iron spoon. Sulfur dioxide is formed - sulfur oxide (4) - SO 2.
S + O 2 = SO 2
Student. The sulfur lights up with a bluish flame, produces abundant acrid smoke, and releases heat and light.
Demonstration experiment No. 6
Teacher. The decomposition reaction of potassium permangate is a reaction for the production and recognition of oxygen.
Student. Gas is released.
Teacher. This reaction occurs with constant heating, as soon as it is stopped, the reaction also stops (the tip of the gas outlet tube of the device where oxygen was obtained is lowered into a test tube with water - while heating, oxygen is released, and it can be seen by the bubbles coming out of the tip of the tube, but if stop heating - the release of oxygen bubbles also stops).
Demonstration experiment No. 7
Teacher. Add a little NaOH alkali to a test tube with NH 4 CL ammonium chloride while heating. Ask one of the students to come up and smell the ammonia released. Warn the student about the strong smell!
NH 4 CL + NaOH = NH 3 + HOH + NaCL
Student. A gas with a pungent odor is released.
Students write down signs of chemical reactions in their notebooks.
Signs of chemical reactions
Release (absorption) of heat or light
Color change
Gas release
Isolation (dissolution) of sediment
Change in smell
Using students’ knowledge of chemical reactions, based on the demonstration experiments performed, we compile a table of the conditions for the occurrence and occurrence of chemical reactions
Teacher. You have studied the signs of chemical reactions and the conditions for their occurrence. Individual work by cards.
Which signs are characteristic of chemical reactions?
A) Formation of sediment
B) Change in state of aggregation
B) Gas release
D) Grinding of substances
Final part
The teacher sums up the lesson, analyzing the results obtained. Gives grades.
Homework
Give examples of chemical phenomena that occur in labor activity your parents, in the household, in nature.
According to the textbook by O.S. Gabrielyan “Chemistry - 8th grade” § 26, ex. 3.6 p.96
1. By characteristic changes in oxidation states of elements in molecules of reacting substances, all reactions are divided into:
A) redox reactions (electron transfer reactions);
b) not redox reactions (reactions without electron transfer).
2. According to the sign of the thermal effect all reactions are divided into:
A) exothermic (coming with the release of heat);
b) endothermic (coming with heat absorption).
3. By characteristic homogeneity of the reaction system reactions are divided into:
A) homogeneous (flowing in homogeneous system);
b) heterogeneous (flowing in a heterogeneous system)
4. Depending on presence or absence of catalyst reactions are divided into:
A) catalytic (coming with the participation of a catalyst);
b) non-catalytic (running without a catalyst).
5. By characteristic reversibility all chemical reactions are divided into:
A) irreversible (flowing in one direction only);
b) reversible (flowing simultaneously in forward and reverse directions).
Let's look at another frequently used classification.
According to the number and composition of starting substances (reagents) and reaction products The following most important types of chemical reactions can be distinguished:
A) connection reactions; b) decomposition reactions;
V) substitution reactions; G) exchange reactions.
Compound reactions- these are reactions during which two or more substances form one substance of a more complex composition:
A + B + ... = B.
Exists large number reactions of combining simple substances (metals with non-metals, non-metals with non-metals), for example:
Fe + S = FeS 2Na + H 2 = 2NaH
S + O 2 = SO 2 H 2 + Cl 2 = 2HCl
Reactions of combining simple substances are always redox reactions. Typically, these reactions are exothermic.
Complex substances can also participate in compound reactions, for example:
CaO + SO 3 = CaSO 4 K 2 O + H 2 O = 2KOH
CaCO 3 + CO 2 + H 2 O = Ca (HCO 3) 2
In the examples given, the oxidation states of elements do not change during the reactions.
There are also reactions of combining simple and complex substances, which belong to redox reactions, for example:
2FeCl 2 + Cl 2 = 2FeCl 3 2SO 2 + O 2 = 2SO 3
· Decomposition reactions- these are reactions in which two or more simpler substances are formed from one complex substance: A = B + C + ...
The decomposition products of the original substance can be both simple and complex substances, for example:
2Fe(OH) 3 = Fe 2 O 3 + 3H 2 O BaCO 3 = BaO + CO 2
2АgNO3 = 2Аg + 2NO2 + О2
Decomposition reactions usually occur when substances are heated and are endothermic reactions. Like compound reactions, decomposition reactions can occur with or without changes in the oxidation states of elements.
Substitution reactions- these are reactions between simple and complex substances, during which the atoms simple substance replace atoms of one of the elements in the molecule of a complex substance. As a result of the substitution reaction, a new simple and a new complex substance are formed:
A + BC = AC + B
These reactions are almost always redox reactions. For example:
Zn + 2HCl = ZnСl 2 + H 2
Ca + 2H 2 O = Ca(OH) 2 + H 2
Fe + CuSO 4 = FeSO 4 + Cu
2Al + Fe 2 O 3 = 2Fe + Al 2 O 3
2KBr + Cl 2 = 2KCl + Br 2
There are a small number of substitution reactions that involve complex substances and that occur without changing the oxidation states of the elements, for example:
CaCO 3 + SiO 2 = CaSiO 3 + CO 2
Ca 3 (PO 4) 2 + 3SiO 2 = 3CaSiO 3 + P 2 O 5
Exchange reactions- these are reactions between two complex substances, the molecules of which exchange their components:
AB + SV = AB + SV
Exchange reactions always occur without electron transfer, i.e. they are not redox reactions. For example:
HNO 3 + NaOH = NaNO 3 + H 2 O
BaCl 2 + H 2 SO 4 = BaSO 4 + 2HCl
As a result of exchange reactions, a precipitate (↓), or a gaseous substance (), or a weak electrolyte (for example, water) is usually formed.
In industry, conditions are selected so that the necessary reactions are carried out and harmful ones are slowed down.
TYPES OF CHEMICAL REACTIONS
Table 12 shows the main types of chemical reactions according to the number of particles involved in them. Drawings and equations of reactions often described in textbooks are given. decomposition, connections, substitution And exchange.
At the top of the table are presented decomposition reactions water and sodium bicarbonate. Shown is a device for passing direct electric current through water. The cathode and anode are metal plates immersed in water and connected to a source of electric current. Due to the fact that pure water practically does not conduct electric current, a small amount of soda (Na 2 CO 3) or sulfuric acid (H 2 SO 4) is added to it. When current passes through both electrodes, gas bubbles are released. In the tube where hydrogen is collected, the volume turns out to be twice as large as in the tube where oxygen is collected (its presence can be verified with the help of a smoldering splinter). The model diagram demonstrates the reaction of water decomposition. Chemical (covalent) bonds between atoms in water molecules are destroyed, and molecules of hydrogen and oxygen are formed from the released atoms.
Model diagram connection reactions metallic iron and molecular sulfur S 8 shows that as a result of rearrangement of atoms during the reaction, iron sulfide is formed. In this case, the chemical bonds in the iron crystal (metallic bond) and the sulfur molecule ( covalent bond), and the released atoms combine to form ionic bonds to form a salt crystal.
Another reaction of the compound is the slaking of lime with CaO with water to form calcium hydroxide. At the same time, burnt (quicklime) lime begins to heat up and loose slaked lime powder is formed.
TO substitution reactions refers to the interaction of a metal with an acid or salt. When a sufficiently active metal is immersed in a strong (but not nitric) acid, hydrogen bubbles are released. More active metal displaces the less active salt from the solution.
Typical exchange reactions is a neutralization reaction and a reaction between solutions of two salts. The figure shows the preparation of barium sulfate precipitate. The progress of the neutralization reaction is monitored using the phenolphthalein indicator (the crimson color disappears).
Table 12
Types of chemical reactions

AIR. OXYGEN. COMBUSTION
Oxygen is the most common chemical element on Earth. Its content in earth's crust and the hydrosphere are presented in Table 2 “Occurrence of chemical elements”. Oxygen accounts for approximately half (47%) of the mass of the lithosphere. It is the predominant chemical element of the hydrosphere. In the earth's crust, oxygen is present only in bound form(oxides, salts). The hydrosphere is also represented mainly by bound oxygen (part of the molecular oxygen is dissolved in water).
The atmosphere contains 20.9% free oxygen by volume. Air is a complex mixture of gases. Dry air consists of 99.9% nitrogen (78.1%), oxygen (20.9%) and argon (0.9%). The content of these gases in the air is almost constant. The composition of the dry atmospheric air also includes carbon dioxide, neon, helium, methane, krypton, hydrogen, nitric oxide (I) (dianitrogen oxide, nitrogen hemioxide - N 2 O), ozone, sulfur dioxide, carbon monoxide, xenon, nitric oxide (IV) (nitrogen dioxide – NO 2).
The composition of air was determined by the French chemist Antoine Laurent Lavoisier at the end of the 18th century (Table 13). He proved the oxygen content in the air and called it “life air.” To do this, he heated mercury on a stove in a glass retort, the thin part of which was placed under a glass cap placed in a water bath. The air under the hood turned out to be closed. When heated, mercury combined with oxygen, turning into red mercuric oxide. The “air” remaining in the glass bell after heating the mercury did not contain oxygen. The mouse, placed under the hood, was suffocating. Having calcined the mercury oxide, Lavoisier again isolated oxygen from it and again obtained pure mercury.
The oxygen content in the atmosphere began to increase noticeably about 2 billion years ago. As a result of the reaction photosynthesis a certain volume of carbon dioxide was absorbed and the same volume of oxygen was released. The figure in the table schematically shows the formation of oxygen during photosynthesis. During photosynthesis in the leaves of green plants containing chlorophyll, when solar energy is absorbed, water and carbon dioxide are converted into carbohydrates(sugar) and oxygen. The reaction of the formation of glucose and oxygen in green plants can be written as follows:
6H 2 O + 6CO 2 = C 6 H 12 O 6 + 6O 2.
The resulting glucose becomes insoluble in water starch, which accumulates in plants.
Table 13
Air. Oxygen. Combustion

Photosynthesis is a complex chemical process that includes several stages: the absorption and transport of solar energy, the use of sunlight energy to initiate photochemical redox reactions, the reduction of carbon dioxide and the formation of carbohydrates.
Sunlight- This electromagnetic radiation different wavelengths. In the chlorophyll molecule, when visible light (red and violet) is absorbed, electrons transition from one energy state to another. Only a small portion of solar energy (0.03%) reaching the Earth's surface is consumed for photosynthesis.
All carbon dioxide on Earth goes through the photosynthesis cycle on average in 300 years, oxygen in 2000 years, and ocean water in 2 million years. Currently, a constant oxygen content has been established in the atmosphere. It is almost completely spent on respiration, combustion and decay of organic substances.
Oxygen is one of the most active substances. Processes involving oxygen are called oxidation reactions. These include combustion, breathing, rotting and many others. The table shows the combustion of oil, which occurs with the release of heat and light.
Combustion reactions can bring not only benefits, but also harm. Combustion can be stopped by cutting off the access of air (oxidizer) to the burning object using foam, sand or a blanket.
Foam fire extinguishers are filled with a concentrated solution of baking soda. When it comes into contact with concentrated sulfuric acid, located in a glass ampoule at the top of the fire extinguisher, a foam of carbon dioxide is formed. To activate the fire extinguisher, turn it over and hit the floor with a metal pin. In this case, the ampoule with sulfuric acid breaks and the resulting reaction of the acid with sodium bicarbonate carbon dioxide foams the liquid and throws it out of the fire extinguisher in a strong stream. Foamy liquid and carbon dioxide, enveloping a burning object, pushes away the air and extinguishes the flame.
Related information.