The structure of the tellurium atom. Tellurium. Description of tellurium. Properties of tellurium Which family does tellurium belong to?
DEFINITION
Tellurium located in the fifth period of the VI group of the main (A) subgroup of the Periodic Table.
Refers to elements p-families. Metalloid. Designation - Te. Serial number - 52. Relative atomic mass - 127.60 amu.
Electronic structure of the tellurium atom
A tellurium atom consists of a positively charged nucleus (+52), inside of which there are 52 protons and 76 neutrons, and 52 electrons move around in five orbits.
Fig.1. Schematic structure of a tellurium atom.
The distribution of electrons among orbitals is as follows:
52Te) 2) 8) 18) 18) 6 ;
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 4 .
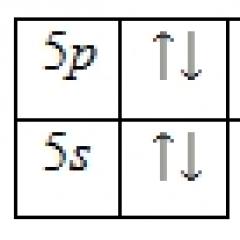
The outer energy level of the tellurium atom contains 6 electrons, which are valence electrons. The energy diagram of the ground state takes the following form:

The valence electrons of a tellurium atom can be characterized by a set of four quantum numbers: n(main quantum), l(orbital), m l(magnetic) and s(spin):
|
Sublevel |
||||
Examples of problem solving
EXAMPLE 1
Thus, for particles S +6, S 0, S +4 and S -2, the total number of electrons in electron shells will be 10, 16, 12 and 18, respectively. Then the table will look like this:
EXAMPLE 2
Thus, for particles C +4, Al +3, F and C 0, the total number of electrons in electron shells will be equal to 2, 10, 10 and 6, respectively. Then the table will look like this:
|
tellurium element, tellurium Wikipedia
Tellurium / Tellurium (Te), 52
(molar mass)
127.60(3) a. e.m. (g/mol)
(+6e) 56,211 (−2e) pm
2.1 (Pauling scale)
6, +4 , +2, −2
(first electron)
869.0 (9.01) kJ/mol (eV)
17.91 kJ/mol
49.8 kJ/mol
25.8 J/(K mol)
20.5 cm³/mol
hexagonal
(300 K) 14.3 W/(mK)
| 52 | |
| Te 127,60 | |
| 4d105s25p4 | |
Tellurium- chemical element of the 16th group (according to the outdated classification - the main subgroup of group VI, chalcogens), 5th period in the periodic table, has atomic number 52; indicated by the symbol Te(lat. Tellurium), belongs to the family of metalloids.
- 1. History
- 2 Origin of the name
- 3 Being in nature
- 3.1 Types of deposits
- 4 Receipt
- 4.1 Prices
- 5 Physical properties
- 6 Chemical properties
- 7 Isotopes
- 8 Application
- 8.1 Alloys
- 8.2 Thermoelectric materials
- 8.3 Narrow-gap semiconductors
- 8.4 High temperature superconductivity
- 8.5 Rubber production
- 8.6 Production of chalcogenide glasses
- 8.7 Light sources
- 8.8 CD-RW
- 9 Biological role
- 9.1 Physiological effect
- 10 Notes
- 11 Links
Story
It was first discovered in 1782 in the gold ores of Transylvania by mining inspector Franz Joseph Müller (later Baron von Reichenstein), on the territory of Austria-Hungary. In 1798, Martin Heinrich Klaproth isolated tellurium and determined its most important properties.
origin of name
From Latin tellus, genitive telluris, Earth.
Being in nature
Content in the earth's crust is 1·10−6% by mass. About 100 tellurium minerals are known. The most common tellurides are copper, lead, zinc, silver and gold. An isomorphic admixture of tellurium is observed in many sulfides, but the Te - S isomorphism is less pronounced than in the Se - S series, and sulfides contain a limited admixture of tellurium. Among tellurium minerals, altaite (PbTe), sylvanite (AgAuTe4), calaverite (AuTe2), hessite (Ag2Te), krennerite, petzite (Ag3AuTe2), mutmannite, montbreuite (Au2Te3), nagiagite (4S5), tetradymite (Bi2Te2S) are of particular importance. There are oxygen compounds of tellurium, for example, TeO2 - tellurium ocher.
Native tellurium also occurs together with selenium and sulfur (Japanese telluric sulfur contains 0.17% Te and 0.06% Se).
Types of deposits
Most of the mentioned minerals are developed in low-temperature gold-silver deposits, where they are usually isolated after the bulk of sulfides together with native gold, sulfosalts of silver, lead, and also with bismuth minerals. Despite the development of a large number of tellurium minerals, the bulk of tellurium extracted by industry is part of sulfides of other metals. In particular, tellurium, to a slightly lesser extent than selenium, is included in the composition of chalcopyrite in copper-nickel deposits of igneous origin, as well as chalcopyrite developed in copper pyrite hydrothermal deposits. Tellurium is also found in pyrite, chalcopyrite, molybdenite and galena of porphyry copper ore deposits, polymetallic deposits of the Altai type, galena of lead-zinc deposits associated with skarns, sulfide-cobalt, antimony-mercury and some others. The tellurium content in molybdenite ranges from 8-53 g/t, in chalcopyrite 9-31 g/t, in pyrite - up to 70 g/t.
Receipt
The main source is sludge from electrolytic refining of copper and lead. The sludge is fired, the tellurium remains in the cinder, which is washed with hydrochloric acid. Tellurium is isolated from the resulting hydrochloric acid solution by passing sulfur dioxide SO2 through it.
Sulfuric acid is added to separate selenium and tellurium. In this case, tellurium dioxide TeO2 falls out, and H2SeO3 remains in solution.
Tellurium is reduced from TeO2 oxide with coal.
To purify tellurium from sulfur and selenium, its ability, under the influence of a reducing agent (Al, Zn) in an alkaline medium, to transform into soluble disodium ditelluride Na2Te2 is used:
To precipitate tellurium, air or oxygen is passed through the solution:
To obtain tellurium of special purity, it is chlorinated
The resulting tetrachloride is purified by distillation or rectification. The tetrachloride is then hydrolyzed with water:
,and the resulting TeO2 is reduced with hydrogen:
Prices
Tellurium is a rare element, and significant demand with a small volume of production determines its high price (about $200–300 per kg, depending on purity), but despite this, the range of its applications is constantly expanding.
Physical properties
Tellurium is a brittle, silvery-white substance with a metallic luster. in thin layers, when exposed to light, red-brown; in pairs, golden-yellow. When heated, it becomes plastic. The crystal lattice is hexagonal. The thermal expansion coefficient is 1.68·10-5 K−1. Diamagnetic. Semiconductor with a band gap of 0.34 eV, type of conductivity - p under normal conditions and at elevated temperatures, n - at low temperatures (transition limit - from minus 80 to minus 100 ° C, depending on purity).
Chemical properties
In chemical compounds, tellurium exhibits oxidation states –2; +2; +4; +6. It is an analogue of sulfur and selenium, but is chemically less active than sulfur. It dissolves in alkalis, is susceptible to the action of nitric and sulfuric acids, but is poorly soluble in dilute hydrochloric acid. Tellurium metal begins to react with water at 100 °C.
With oxygen it forms compounds TeO, TeO2, TeO3. in powder form, it oxidizes in air even at room temperature, forming TeO2 oxide. When heated in air, it burns, forming TeO2 - a strong compound that is less volatile than tellurium itself. This property is used to purify tellurium from oxides, which are reduced with flowing hydrogen at a temperature of 500-600 °C. Tellurium dioxide is poorly soluble in water, but soluble in acidic and alkaline solutions.
In the molten state, tellurium is quite inert, so graphite and quartz are used as container materials when melting it.
Tellurium forms a compound with hydrogen when heated, easily reacts with halogens, and interacts with sulfur and phosphorus and metals. When reacting with concentrated sulfuric acid, it forms sulfite. Forms weak acids: hydrotelluric acid (H2Te), telluric acid (H2TeO3) and telluric acid (H6TeO6), most of whose salts are poorly soluble in water.
Isotopes
Main article: Isotopes of telluriumThere are 38 known nuclides and 18 nuclear isomers of tellurium with atomic numbers from 105 to 142. Tellurium is the lightest element, whose known isotopes are subject to alpha decay (isotopes from 106Te to 110Te). The atomic mass of tellurium (127.60 g/mol) exceeds the atomic mass of the next element, iodine (126.90 g/mol).
Eight isotopes of tellurium occur in nature. Six of them, 120Te, 122Te, 123Te, 124Te, 125Te and 126Te, are stable. The remaining two - 128Te and 130Te - are radioactive, both of them undergo double beta decay, turning into xenon isotopes 128Xe and 130Xe, respectively. Stable isotopes make up only 33.3% of the total amount of tellurium found in nature, which is made possible by the extremely long half-lives of natural radioactive isotopes. They range from 7.9·1020 to 2.2·1024 years. The isotope 128Te has the longest confirmed half-life of any radionuclide - 2.2 1024 years or 2.2 septillion years, which is about 160 trillion times the estimated age of the Universe.
Application
Alloys
Tellurium is used in the production of lead alloys with increased ductility and strength (used, for example, in the production of cables). With the introduction of 0.05% tellurium, the loss of lead due to dissolution under the influence of sulfuric acid is reduced by 10 times, and this is used in the production of lead-acid batteries. It is also important that lead alloyed with tellurium does not soften when processed by plastic deformation, and this makes it possible to use the technology for manufacturing battery plate current collectors using the cold cutting method and significantly increase the service life and specific characteristics of the battery.
Thermoelectric materials
Bismuth telluride single crystalIts role is also great in the production of semiconductor materials and, in particular, tellurides of lead, bismuth, antimony, and cesium. In the coming years, the production of lanthanide tellurides, their alloys and alloys with metal selenides will become very important for the production of thermoelectric generators with very high (up to 72-78%) efficiency, which will make it possible to use them in the energy sector and in the automotive industry.
For example, a very high thermal emf was recently discovered in manganese telluride (500 μV/K) and in its combination with bismuth, antimony and lanthanide selenides, which makes it possible not only to achieve a very high efficiency in thermogenerators, but also to implement it in one stage semiconductor refrigerator cooling down to the cryogenic (temperature level of liquid nitrogen) temperatures and even lower. The best tellurium-based material for the production of semiconductor refrigerators in recent years has been an alloy of tellurium, bismuth and cesium, which made it possible to obtain record cooling down to −237 °C. At the same time, as a thermoelectric material, a tellurium-selenium alloy (70% selenium), which has a thermo-EMF coefficient of about 1200 μV/K, is promising.
Narrow-gap semiconductors
CRT (cadmium-mercury-tellurium) alloys have also received absolutely exceptional importance, which have fantastic characteristics for detecting radiation from rocket launches and observing the enemy from space through atmospheric windows (cloud cover does not matter). MCT is one of the most expensive materials in the modern electronics industry.
High temperature superconductivity
A number of systems containing tellurium have recently discovered the existence in them of three (possibly four) phases, in which superconductivity does not disappear at a temperature slightly above the boiling point of liquid nitrogen.
Rubber production
A separate area of application for tellurium is its use in the process of rubber vulcanization.
Production of chalcogenide glasses
Tellurium is used in the melting of special grades of glass (where it is used in the form of dioxide); special glasses doped with rare earth metals are used as active bodies in optical quantum generators.
In addition, some tellurium-based glasses are semiconductors, a property that is used in electronics.
Special grades of tellurium glass (the advantages of such glass are transparency, fusibility and electrical conductivity) are used in the construction of special chemical equipment (reactors).
Sources of light
Tellurium finds limited use in the production of lamps with its vapors - they have a spectrum very close to that of the sun.
CD-RW
Tellurium alloy is used in rewritable compact discs (in particular, Mitsubishi Chemical Corporation brand "Verbatim") to create a deformable reflective layer.
Biological role
Micro amounts of tellurium are always found in living organisms; its biological role is not clear.
Physiological action
Tellurium and its volatile compounds are toxic. If it enters the body it causes nausea, bronchitis, and pneumonia. MPC in air varies for various compounds 0.007-0.01 mg/m³, in water 0.001-0.01 mg/l. The carcinogenicity of tellurium has not been confirmed.
In general, tellurium compounds are less toxic than selenium compounds.
In case of poisoning, tellurium is excreted from the body in the form of foul-smelling volatile organotellurium compounds - alkyl tellurides, mainly dimethyl telluride (CH3)2Te. Their smell is reminiscent of garlic, so when even small amounts of tellurium enter the body, the air exhaled by a person acquires this smell, which is an important symptom of tellurium poisoning.
Notes
- Michael E. Wieser, Norman Holden, Tyler B. Coplen, John K. Böhlke, Michael Berglund, Willi A. Brand, Paul De Bièvre, Manfred Gröning, Robert D. Loss, Juris Meija, Takafumi Hirata, Thomas Prohaska, Ronny Schoenberg, Glenda O'Connor, Thomas Walczyk, Shige Yoneda, Xiang-Kun Zhu. Atomic weights of the elements 2011 (IUPAC Technical Report) // Pure and Applied Chemistry. - 2013. - Vol. 85, no. 5. - P. 1047-1078. - DOI:10.1351/PAC-REP-13-03-02.
- Tellurium: electronegativities (English). WebElements. Retrieved August 5, 2010.
- Leddicotte, G. W. (1961), “The radiochemistry of tellurium,” Nuclear science series, Subcommittee on Radiochemistry, National Academy of Sciences-National Research Council, p. 5,
- Editorial team: Zefirov N. S. (chief editor). Chemical encyclopedia: in 5 volumes - Moscow: Soviet Encyclopedia, 1995. - T. 4. - P. 514. - 639 p. - 20,000 copies. - ISBN 5-85270-039-8.
- WebElements Periodic Table of the Elements | Tellurium | crystal structures
- Glinka N. L. General chemistry. - M.: “Chemistry”, 1977, revised. - P. 395. - 720 p.
- 1 2 3 4 Tellurium - article from the Great Soviet Encyclopedia
- 1 2 G. Audi, O. Bersillon, J. Blachot and A. H. Wapstra (2003). "The NUBASE evaluation of nuclear and decay properties." Nuclear Physics A 729 : 3–128. DOI:10.1016/j.nuclphysa.2003.11.001. Bibcode: 2003NuPhA.729....3A.
- The tellurium-123 isotope was considered radioactive (β−-active with a half-life of 6·1014 years), but after additional measurements it was found to be stable within the sensitivity of the experiment.
- 2.2 quadrillion years - on the long scale.
- Tellurium. International Program on Chemical Safety (28 January 1998). Retrieved January 12, 2007. Archived from the original on August 4, 2012.
- Wright, P. L. (1966). "Comparative metabolism of selenium and tellurium in sheep and swine". AJP – Legacy 211 (1): 6–10. PMID 5911055.
- (1989) "Tellurium-intoxication". Klinische Wochenschrift 67 (22): 1152–5. DOI:10.1007/BF01726117. PMID 2586020.
- Taylor, Andrew (1996). "Biochemistry of tellurium". Biological Trace Element Research 55 (3): 231–239. DOI:10.1007/BF02785282. PMID 9096851.
Links
- Tellurium on Webelements
- Tellurium at the Popular Chemical Elements Library
| Connections tellurium | |
|---|---|
|
Tellurium hexafluoride (TeF6) Tellurium dioxide (TeO2) Sodium orthotellurate (Na6TeO6) Ammonium tellurate ((NH4)2TeO4) Beryllium telluride (BeTe) Bismuth(III) telluride (Bi2Te3) Dipotassium telluride (K2Te) Cadmium telluride (CdTe) Sodium telluride (Na2Te ) Tin telluride (SnTe) Mercury telluride (HgTe) Lead telluride (PbTe) Zinc telluride (ZnTe) Potassium tellurite (K2TeO3) Sodium tellurite (Na2TeO3) Telluric acid (H2TeO4 2H2O) Hydrogen telluride (H2Te) Tellurophene C4H4Te Tetra tellurium bromide (TeBr4) Potassium tetrahydroorthotellurate K2H4TeO6 Tellurium tetraiodide (TeI4) Tellurium tetrafluoride (TeF4) Tellurium tetrachloride (TeCl4) Tellurium trioxide (TeO3) Dipotassium tritelluride (K2Te3) |
| Periodic table of chemical elements by D. I. Mendeleev | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | MD | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
| 8 | Uue | Ubn | Ubu | Ubb | Ubt | Ubq | UBP | Ubh | ||||||||||||||||||||||||
tellurium Wikipedia, tellurium Kharkov, tellurium element, tellurium, tellurium photo, tellurite, tellurium, tellurium sorokin, tellurium sorokin download, tellurocracy
Tellurium Information About
Tellurium is a chemical element of group 16 (according to the outdated classification - the main subgroup of group VI, chalcogens), period 5 in the periodic table, has atomic number 52; denoted by the symbol Te (lat. Tellurium), belongs to the family of metalloids.
Content in the earth's crust is 1·10-6% by weight. About 100 tellurium minerals are known. The most common tellurides are copper, lead, zinc, silver and gold.
An isomorphic admixture of tellurium is observed in many sulfides, but the Te - S isomorphism is less pronounced than in the Se - S series, and sulfides contain a limited admixture of tellurium. Among tellurium minerals, altaite (PbTe), sylvanite (AgAuTe4), calaverite (AuTe2), hessite (Ag2Te), krennerite [(Au, Ag)Te], petzite (Ag3AuTe2), muthmannite [(Ag, Au)Te] are of particular importance , montbreuite (Au2Te3), nagiagite (4S5), tetradymite (Bi2Te2S). There are oxygen compounds of tellurium, for example TeO2 - tellurium ochre. Native tellurium also occurs together with selenium and sulfur (Japanese telluric sulfur contains 0.17% Te and 0.06% Se).
Reserves at tellurium deposits in 2012, tons *
| Peru | 3,600.0 |
| USA | 3,500.0 |
| Canada | 800.0 |
| Other countries | 16,100.0 |
| Total stocks | 24,000.0 |
* US Geological Survey data
The main source of tellurium is sludge produced during the electrolytic purification of blister (anodic) copper. For every 500 tons of copper ore, there is typically one pound (0.45 kg) of tellurium. Tellurium is produced primarily in the United States, China, Belgium, Russia, Japan and Canada.
Anode slurry contains selenides and tellurides of noble metals in compositions with the formula M2Se or M2Te (M = Cu, Ag, Au). At temperatures of 500 °C, the anode sludge is heated with sodium carbonate in the presence of air. Metal ions are reduced to metals while telluride is converted to sodium tellurite - M2Te + O2 + Na2CO3 > Na2TeO3 + 2M + CO2.
Tellurites leach from mixtures with water and are usually present as hydrotellurites HTeO3– in solution. Selenites are also formed during this process, but they can be separated by adding sulfuric acid. Hydrotellurites are transformed into insoluble tellurium dioxide, while selenites remain in solution - HTeO3- + ОH– + H2SO4 > TeO2 + SO42- + 2H2O.
Reduction to metal is done either by electrolysis or by the reaction of tellurium dioxide with sulfur dioxide in sulfuric acid - TeO2 + 2 SO2 + 2H2O > Te + SO42- + 4H+.
Commercial grade tellurium is usually sold as a powder and is also available in the form of slabs, ingots, or rods.
The largest consumer of tellurium is metallurgy, where it is used in iron, copper and lead alloys. Adding tellurium to stainless steel and copper makes these metals more workable. The addition of tellurium makes it possible to obtain malleable cast iron, which, when smelted, has the advantages of gray cast iron: liquid casting, casting properties, and machinability. In lead, tellurium improves strength and durability and reduces the corrosive effect of sulfuric acid.
Semiconductors and electronics. Cadmium telluride (CdTe) is used in solar cells. Tests by the Renewable Energy Laboratory in the United States have shown that this material provides many benefits for the operation of a new generation of solar cells. Massive commercial production of solar cells using CdTe in recent years has led to a significant increase in demand for tellurium. If some of the cadmium in CdTe is replaced by zinc, the ratio (Cd,Zn) is formed, which is used in solid state X-ray sensors.
CRT (cadmium-mercury-tellurium) alloys have received absolutely exceptional importance, which have fantastic characteristics for detecting radiation from rocket launches and observing the enemy from space through atmospheric windows (cloud cover does not matter). MCT is one of the most expensive materials in the modern electronics industry.
Organotelluride such as ethane telluride, diethyl telluride, diisopropyl telluride, diethyl and methyl telluride, allyl telluride are used as the basis for organometallic growth phase epitaxy to produce multilayer semiconductor compounds.
A number of systems containing tellurium have recently discovered the existence in them of three (possibly four) phases, in which superconductivity does not disappear at a temperature slightly above the boiling point of liquid nitrogen.
Tellurium as tellurium oxide is used to create layers of rewritable optical discs, including Compact Discs ReWritable (CD-RW), ReWritable Blu-ray Digital Video Discs and ReWritable (DVD-RW).
Tellurium is used in new phase change memory chips developed by Intel. Bismuth telluride (Bi2Te3) and lead telluride are used in elements of thermoelectric devices. Lead telluride is also used in infrared sensors.
Other uses. Tellurium is used to color ceramics. The phenomenon of a strong increase in optical refraction after adding selenides and tellurides to glass is used in the production of glass fibers for telecommunications. Mixtures of selenium and tellurium are used with barium peroxide as an oxidizing agent in delay powder for electric blasting caps.
Organic tellurides are used as initiators for radical polymerization; electron-rich mono- and ditellurides have antioxidant activity. Tellurium can be used instead of sulfur or selenium to vulcanize rubber. Rubber produced in this way exhibits improved thermal resistance. Tellurites are used to identify the pathogens responsible for diphtheria.
Tellurium consumption in countries around the world is distributed as follows: China - 80-100 tons, Russia - 10 tons, USA - 50-60 tons. In total, about 400 tons of tellurium are consumed annually in the world as a whole. The table below provides approximate data on tellurium production in the world (data from the USGS, various reviews and articles on the market).
Tellurium production in the world, tons*
| year | 2008 | 2009 | 2010 | 2011 | 2012 |
| Belgium | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Canada | 19.0 | 16.0 | 8.0 | 6.0 | 6.0 |
| China | 65.0 | 60.0 | 65.0 | 70.0 | 70.0 |
| Japan | 46.5 | 49.2 | 47.0 | 40.0 | 35.0 |
| Kazakhstan | 18.0 | 17.0 | 18.0 | 18.0 | 17.0 |
| Peru | 28.0 | 7.0 | -- | -- | -- |
| Russia | 34.0 | 33.0 | 34.0 | 34.0 | 35.0 |
| USA | 50.0 | 50.0 | 50.0 | 50.0 | 45.0 |
| Other countries | 79.5 | 97.8 | 128.0 | 132.0 | 122.0 |
| Total | 390.0 | 380.0 | 400.0 | 400.0 | 380.0 |
* US Geological Survey data
Tellurium is a rare element, and significant demand with a small volume of production determines its high price (about $200-300 per kg, depending on purity), but despite this, the range of its applications is constantly expanding.
The price of tellurium in 2000 was about US$30 per kilogram. Between 2004 and 2011, tellurium prices increased continuously, with the exception of 2009. During these years, the price of tellurium was determined by a significant increase in demand and limited supply. In 2011, the price of tellurium reached US$350 per kilogram. However, in 2012, tellurium prices fell sharply to approximately US$150 per kilogram.
The tellurium market currently faces a number of challenges. As a by-product of copper production, the tellurium market is highly dependent on trends in the main (copper) market. A decrease in copper production along with the use of new alternative technologies for producing this metal, for example, will affect the supply volumes of tellurium.
As supply volumes are in doubt, the price of the material is skyrocketing. According to many market forecasts, the price of tellurium will rise again in the next 2-3 years. It is known that there is a range of different tellurium replacement products on the market, which are already beginning to be used amid supply shortages. However, as experts note, none of the replacements has the same properties as tellurium. In addition, a potential increase in demand for tellurium could result from developments in the solar thin film sector.
Tellurium – chemical element belonging to the 16th group, located in the periodic table, atomic number 52 and designated by the Latin Te - special identification. The element belongs to the metalloids. Tellurium formula — 4d10 5s2 5p4.
Tellurium - element having a white-silver tint and a metallic luster and fragile structure. At high temperatures, like many metals, tellurium becomes ductile.
Origin of tellurium
The element was discovered in gold mines in the mountains of Transylvania. Humanity knows at least one hundred minerals containing tellurium. In particular, these are silver, gold, copper and zinc. There are various tellurium compounds, for example, these are some types of ocher. In its pure form, in one deposit you can find selenium, tellurium and sulfur, which indicates the possibility of the element being native.

All of the minerals mentioned are more often found in the same deposit with silver, lead and bismuth. In industrial settings, for the most part tellurium is isolated chemically from other metals, despite the fact that its main minerals are quite common. In particular, it is contained in sufficient quantities in chalcopyrite, which is part of nickel-copper and copper pyrite ores.
Additionally, it can be found in, molybdenite and galena, it is also found in copper ores, polymetallic deposits and lead-zinc deposits. These minerals also contain sulfide and antimony rocks containing cobalt and mercury.
Mostly in industry, tellurium is extracted from sludge, which is formed by the electrolytic refining of copper and lead. During processing, the sludge is burned, and the burnt residues contain a certain tellurium content. To isolate the required element, the cinders are washed with hydrochloric acid.

To separate the metal from the resulting acid solution, sulfur dioxide must be passed through it. Obtained in this way tellurium oxide, is processed with coal to obtain a pure element from it. For its further purification, a chlorination procedure is used.
This produces tetrachloride, which must be purified by distillation or rectification. Next, it is hydrolyzed, and the resulting tellurium hydroxide is reduced by hydrogen.
Applications of tellurium
This metal is used in the manufacture of many different materials (copper, lead, iron), so the metallurgy industry is its main consumer. Tellurium makes stainless steel and copper more workable. Also, adding this element to malleable cast iron gives it the positive properties of gray cast iron.
Its casting qualities and machinability are improved. It is able to significantly improve the physical properties of lead, reducing negative corrosion from sulfuric acid during its processing.

Tellurium is widely used in semiconductor devices and electronics. In particular, it is used to produce solar cells. The use of tellurium opens up broad prospects in the application of these advanced technologies. The percentage of production of such equipment has increased significantly in recent years. This led to a noticeable increase in the turnover of tellurium on the world market.
The metal is used, including in space technological developments, in particular, these are alloys with the addition of tellurium, which have unique properties. They are used in technologies for detecting radiation left by spacecraft.
For this reason, the expensive alloy is largely in demand in the military industry, for tracking the enemy in outer space. In addition to this mixture selenium – tellurium is part of the delay powder in detonator caps for explosive devices produced by military factories.

Various tellurium compounds are used in the production of semiconductor compounds with a multilayer structure. Many compounds that include tellurium exhibit remarkable superconductivity.
Tellurium also works for the benefit of ordinary people. In particular, metal oxide is used in the production of compact discs to create a rewritable thin layer on them. It is also present in some microcircuits, for example, those produced by Intel. Bismuth telluride is included in many thermoelectric devices and infrared sensors.
This metal is also used when painting ceramic products. In the manufacture of fiberglass for information communications (television, Internet, etc.), the participation of tellurium in cable production is based on the positive property of tellurides and selenides to increase optical refraction when added to glass.

Vulcanization of rubber also involves the use of substances close to metal - selenium or sulfur, which can be replaced, if possible, by tellurium. Rubber with its addition will demonstrate much better qualities. Tellurium has also found its niche in medicine - it is used in the diagnosis of diphtheria.
Tellurium price
In terms of consumption of this rare earth metal in the world, China is in first place, Russia is in second, and the USA is in third. Total consumption is 400 tons of metal per year. Tellurium is usually sold in the form of powder, rods or.
Due to the small volumes of production, due to its relatively small content in rocks, the price of tellurium is quite high. Approximately, if you do not take into account the constant price hikes for tellurium, buy It can be sold on the world market for $200-300 per kilogram of metal. The price also depends on the degree of purification of the metal from unwanted impurities.
But, despite the inaccessibility of this unique element, there is always considerable demand for it, with constant growth trends. Every year the range of areas requiring the use of tellurium and its compounds is expanding.
It is easy to follow the trend of rising prices for tellurium by comparing prices at the beginning of 2000, when it was $30 per 1 kg, and ten years later, when it reached $350. And despite the fact that a year later it still fell, there is a serious tendency for prices to rise, due to a fall in tellurium production volumes.

The fact is that the tellurium market directly depends on the volume of production, since tellurium is one of the by-products during its extraction. At the moment, the copper market has significantly reduced its turnover, and new technologies for its production have appeared, the features of which will significantly affect the volume of additional tellurium produced.
This will certainly affect its supplies, and naturally prices. According to estimates, a new price hike is expected in a couple of years. Despite the fact that tellurium has certain analogues in industry, they do not have such valuable properties.
This situation on the world market is not at all beneficial for many manufacturers whose production involves tellurium. In particular, these are manufacturers of solar panels, whose products have been gaining increasing popularity in recent years.
17.91 kJ/mol
49.8 kJ/mol
hexagonal
a =4,457 c =5,929
(300 K) 14.3 W/(mK)
Native tellurium also occurs together with selenium and sulfur (Japanese telluric sulfur contains 0.17% Te and 0.06% Se).
Types of deposits
Most of the minerals mentioned are developed in low-temperature gold-silver deposits, where they are usually isolated after the bulk of sulfides together with native gold, sulfosalts of silver, lead, and also with bismuth minerals. Despite the development of a large number of tellurium minerals, the bulk of tellurium extracted by industry is part of sulfides of other metals. In particular, tellurium, to a slightly lesser extent than selenium, is included in the composition of chalcopyrite in copper-nickel deposits of igneous origin, as well as chalcopyrite developed in copper pyrite hydrothermal deposits. Tellurium is also found in pyrite, chalcopyrite, molybdenite and galena of porphyry copper ore deposits, polymetallic deposits of the Altai type, galena of lead-zinc deposits associated with skarns, sulfide-cobalt, antimony-mercury and some others. The tellurium content in molybdenite ranges from 8-53 g/t, in chalcopyrite 9-31 g/t, in pyrite - up to 70 g/t.
Receipt
Chemical properties
In chemical compounds, tellurium exhibits oxidation states –2; +2; +4; +6. It is an analogue of sulfur and selenium, but is chemically less active than sulfur. It dissolves in alkalis, is susceptible to the action of nitric and sulfuric acids, but is poorly soluble in dilute hydrochloric acid. Tellurium metal begins to react with water at 100 °C.
With oxygen it forms compounds TeO, TeO 2, TeO 3. In powder form, it oxidizes in air even at room temperature, forming TeO 2 oxide. When heated in air, it burns, forming TeO 2 - a strong compound that is less volatile than tellurium itself. This property is used to purify tellurium from oxides, which are reduced with flowing hydrogen at a temperature of 500-600 °C. Tellurium dioxide is poorly soluble in water, but soluble in acidic and alkaline solutions.
In the molten state, tellurium is quite inert, so graphite and quartz are used as container materials when melting it.
Tellurium forms a compound with hydrogen when heated, easily reacts with halogens, and interacts with sulfur, phosphorus and metals. When reacting with concentrated sulfuric acid, it forms sulfite. Forms weak acids: hydrotelluric acid (H 2 Te), telluric acid (H 2 TeO 3) and telluric acid (H 6 TeO 6), most of whose salts are poorly soluble in water.
Isotopes
Application
Alloys
Tellurium is used in the production of lead alloys with increased ductility and strength (used, for example, in the production of cables). With the introduction of 0.05% tellurium, the loss of lead due to dissolution under the influence of sulfuric acid is reduced by 10 times, and this is used in the production of lead-acid batteries. It is also important that lead alloyed with tellurium does not soften when processed by plastic deformation, and this makes it possible to use the technology for manufacturing battery plate current collectors using the cold cutting method and significantly increase the service life and specific characteristics of the battery.
The alloy composition CZT (cadmium zinc telluride, CdZnTe) is used in the production of X-ray and gamma radiation detectors that operate at room temperature.
Thermoelectric materials
Its role is also great in the production of semiconductor materials and, in particular, tellurides of lead, bismuth, antimony, and cesium. In the coming years, the production of lanthanide tellurides, their alloys and alloys with metal selenides will become very important for the production of thermoelectric generators with very high (up to 72-78%) efficiency, which will make it possible to use them in the energy sector and in the automotive industry.
For example, a very high thermal emf was recently discovered in manganese telluride (500 μV/K) and in its combination with bismuth, antimony and lanthanide selenides, which makes it possible not only to achieve a very high efficiency in thermogenerators, but also to implement it in one stage semiconductor refrigerator cooling down to the cryogenic (temperature level of liquid nitrogen) temperatures and even lower. The best tellurium-based material for the production of semiconductor refrigerators in recent years has been an alloy of tellurium, bismuth and cesium, which made it possible to obtain record cooling down to −237 °C. At the same time, tellurium-selenium alloy (70% selenium), which has a thermo-EMF coefficient of about 1200 μV/K, is promising as a thermoelectric material.
Narrow-gap semiconductors
CRT (cadmium - tellurium) alloys have also received absolutely exceptional importance, which have fantastic characteristics for detecting radiation from rocket launches and observing the enemy from space through atmospheric windows (cloud cover does not matter). MCT is one of the most expensive materials in the modern electronics industry.
High temperature superconductivity
A number of systems containing tellurium have recently discovered the existence in them of three (possibly four) phases, in which superconductivity does not disappear at a temperature slightly above the boiling point of liquid nitrogen.
Rubber production
A separate area of application for tellurium is its use in the process of rubber vulcanization.
Production of chalcogenide glasses
Tellurium is used in the melting of special grades of glass (where it is used in the form of dioxide); special glasses doped with rare earth metals are used as active bodies in optical quantum generators.
In addition, some tellurium-based glasses are semiconductors, a property that is used in electronics.
Special grades of tellurium glass (the advantages of such glass are transparency, fusibility and electrical conductivity) are used in the construction of special chemical equipment (reactors).
Tellurium finds limited use in the production of lamps with its vapors - they have a spectrum very close to that of the sun.
CD-RW
Tellurium alloy is used in rewritable compact discs (in particular, Mitsubishi Chemical Corporation brand "Verbatim") to create a deformable reflective layer.
Biological role
Physiological action
Tellurium and its volatile compounds are toxic. If it enters the body it causes nausea, bronchitis, and pneumonia. MPC in air varies for various compounds 0.007-0.01 mg/m³, in water 0.001-0.01 mg/l. The carcinogenicity of tellurium has not been confirmed.
In case of poisoning, tellurium is excreted from the body in the form of foul-smelling volatile organotellurium compounds - alkyl tellurides, mainly dimethyl telluride (CH 3) 2 Te. Their smell resembles the smell of garlic, therefore, when even small amounts of tellurium enter the body, the air exhaled by a person acquires this smell, which is an important symptom of tellurium poisoning.
Write a review about the article "Tellurium"
Notes
- Michael E. Wieser, Norman Holden, Tyler B. Coplen, John K. Böhlke, Michael Berglund, Willi A. Brand, Paul De Bièvre, Manfred Gröning, Robert D. Loss, Juris Meija, Takafumi Hirata, Thomas Prohaska, Ronny Schoenberg, Glenda O'Connor, Thomas Walczyk, Shige Yoneda, Xiang-Kun Zhu.(English) // Pure and Applied Chemistry. - 2013. - Vol. 85, no. 5 . - P. 1047-1078. - DOI:10.1351/PAC-REP-13-03-02.
- (English) . WebElements. Retrieved August 5, 2010.
- Leddicotte, G. W. (1961),
, Nuclear science series, Subcommittee on Radiochemistry, National Academy of Sciences-National Research Council, p. 5 ,
- Editorial team: Zefirov N. S. (chief editor). Chemical encyclopedia: in 5 volumes - Moscow: Soviet Encyclopedia, 1995. - T. 4. - P. 514. - 639 p. - 20,000 copies. - ISBN 5-85270-039-8.
- Glinka N. L. General chemistry. - M.: “Chemistry”, 1977, revised. - P. 395. - 720 p.
- Tellurium- article from the Great Soviet Encyclopedia
- G. Audi, O. Bersillon, J. Blachot and A. H. Wapstra (2003). "". Nuclear Physics A 729 : 3–128. DOI:10.1016/j.nuclphysa.2003.11.001. Bibcode:.
- The tellurium-123 isotope was considered radioactive (β − -active with a half-life of 6·10 14 years), but after additional measurements it was found to be stable within the sensitivity of the experiment.
- 2.2 quadrillion years - on the long scale.
- . International Program on Chemical Safety (28 January 1998). Retrieved January 12, 2007. .
- Wright, P. L. (1966). "". AJP – Legacy 211 (1): 6–10. PMID 5911055.
- (1989) "Tellurium-intoxication". Klinische Wochenschrift 67 (22): 1152–5. DOI:10.1007/BF01726117. PMID 2586020.
- Taylor, Andrew (1996). "Biochemistry of tellurium". Biological Trace Element Research 55 (3): 231–239. DOI:10.1007/BF02785282. PMID 9096851.
Links
| Periodic table of chemical elements by D. I. Mendeleev | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Te | ||||||||||||||||||||||||||||||||
| Alkaline earth metals | ||||||||||||||||||||||||||||||||
Excerpt characterizing Tellurium
In the evening, Napoleon, between two orders - one about delivering the prepared counterfeit Russian banknotes for import into Russia as soon as possible, and the other about shooting the Saxon, in whose intercepted letter information about orders for the French army was found - made a third order - about the inclusion of the Polish colonel, who unnecessarily threw himself into the river, into the cohort of honor (Legion d'honneur), of which Napoleon was the head.Qnos vult perdere – dementat. [Whoever he wants to destroy, he will deprive him of his mind (lat.)]
Meanwhile, the Russian emperor had already lived in Vilna for more than a month, making reviews and maneuvers. Nothing was ready for the war that everyone expected and for which the emperor came from St. Petersburg to prepare. There was no general plan of action. Hesitation about which plan, out of all those that were proposed, should be adopted, only intensified even more after the emperor's month-long stay in the main apartment. The three armies each had a separate commander-in-chief, but there was no common commander over all the armies, and the emperor did not assume this title.
The longer the emperor lived in Vilna, the less and less they prepared for war, tired of waiting for it. All the aspirations of the people surrounding the sovereign seemed to be aimed only at making the sovereign, while having a pleasant time, forget about the upcoming war.
After many balls and holidays among the Polish magnates, among the courtiers and the sovereign himself, in June one of the Polish general adjutants of the sovereign came up with the idea of giving a dinner and ball to the sovereign on behalf of his general adjutants. This idea was joyfully accepted by everyone. The Emperor agreed. The general's adjutants collected money by subscription. The person who could be most pleasing to the sovereign was invited to be the hostess of the ball. Count Bennigsen, a landowner of the Vilna province, offered his country house for this holiday, and on June 13 a dinner, ball, boat ride and fireworks display were scheduled at Zakret, Count Bennigsen's country house.
On the very day on which Napoleon gave the order to cross the Neman and his advanced troops, pushing back the Cossacks, crossed the Russian border, Alexander spent the evening at Bennigsen’s dacha - at a ball given by the general’s adjutants.
It was a cheerful, brilliant holiday; experts in the business said that rarely so many beauties gathered in one place. Countess Bezukhova, along with other Russian ladies who came for the sovereign from St. Petersburg to Vilna, was at this ball, darkening the sophisticated Polish ladies with her heavy, so-called Russian beauty. She was noticed, and the sovereign honored her with a dance.
Boris Drubetskoy, en garcon (a bachelor), as he said, having left his wife in Moscow, was also at this ball and, although not an adjutant general, was a participant for a large sum in the subscription for the ball. Boris was now a rich man, far advanced in honor, no longer seeking patronage, but standing on an even footing with the highest of his peers.
At twelve o'clock at night they were still dancing. Helen, who did not have a worthy gentleman, herself offered the mazurka to Boris. They sat in the third pair. Boris, coolly looking at Helen's shiny bare shoulders protruding from her dark gauze and gold dress, talked about old acquaintances and at the same time, unnoticed by himself and others, never for a second stopped watching the sovereign, who was in the same hall. The Emperor did not dance; he stood in the doorway and stopped first one or the other with those gentle words that he alone knew how to speak.
At the beginning of the mazurka, Boris saw that Adjutant General Balashev, one of the closest persons to the sovereign, approached him and stood un-courtly close to the sovereign, who was speaking with a Polish lady. After talking with the lady, the sovereign looked questioningly and, apparently realizing that Balashev acted this way only because there were important reasons, nodded slightly to the lady and turned to Balashev. As soon as Balashev began to speak, surprise was expressed on the sovereign’s face. He took Balashev by the arm and walked with him through the hall, unconsciously clearing three fathoms of wide road on both sides of those who stood aside in front of him. Boris noticed Arakcheev's excited face while the sovereign walked with Balashev. Arakcheev, looking from under his brows at the sovereign and snoring his red nose, moved out of the crowd, as if expecting that the sovereign would turn to him. (Boris realized that Arakcheev was jealous of Balashev and was dissatisfied that some obviously important news was not conveyed to the sovereign through him.)
But the sovereign and Balashev walked, without noticing Arakcheev, through the exit door into the illuminated garden. Arakcheev, holding his sword and looking around angrily, walked about twenty paces behind them.
While Boris continued to make mazurka figures, he was constantly tormented by the thought of what news Balashev had brought and how to find out about it before others.
In the figure where he had to choose ladies, whispering to Helen that he wanted to take Countess Pototskaya, who seemed to have gone out onto the balcony, he, sliding his feet along the parquet floor, ran out the exit door into the garden and, noticing the sovereign entering the terrace with Balashev , paused. The Emperor and Balashev headed towards the door. Boris, in a hurry, as if not having time to move away, respectfully pressed himself against the lintel and bowed his head.
With the emotion of a personally insulted man, the Emperor finished the following words:
- Enter Russia without declaring war. “I will make peace only when not a single armed enemy remains on my land,” he said. It seemed to Boris that the sovereign was pleased to express these words: he was pleased with the form of expression of his thoughts, but was dissatisfied with the fact that Boris heard them.
- So that no one knows anything! – the sovereign added, frowning. Boris realized that this applied to him, and, closing his eyes, bowed his head slightly. The Emperor again entered the hall and remained at the ball for about half an hour.
Boris was the first to learn the news about the crossing of the Neman by French troops and thanks to this he had the opportunity to show some important persons that he knew many things hidden from others, and through this he had the opportunity to rise higher in the opinion of these persons.
The unexpected news about the French crossing the Neman was especially unexpected after a month of unfulfilled anticipation, and at a ball! The Emperor, at the first minute of receiving the news, under the influence of indignation and insult, found what later became famous, a saying that he himself liked and fully expressed his feelings. Returning home from the ball, the sovereign at two o'clock in the morning sent for secretary Shishkov and ordered to write an order to the troops and a rescript to Field Marshal Prince Saltykov, in which he certainly demanded that the words be placed that he would not make peace until at least one the armed Frenchman will remain on Russian soil.
The next day the following letter was written to Napoleon.
“Monsieur mon frere. J"ai appris hier que malgre la loyaute avec laquelle j"ai maintenu mes engagements envers Votre Majeste, ses troupes ont franchis les frontieres de la Russie, et je recois a l"instant de Petersbourg une note par laquelle le comte Lauriston, pour cause de cette aggression, annonce que Votre Majeste s"est consideree comme en etat de guerre avec moi des le moment ou le prince Kourakine a fait la demande de ses passeports. Les motifs sur lesquels le duc de Bassano fondait son refus de les lui delivrer, n "auraient jamais pu me faire supposer que cette demarche servirait jamais de pretexte a l" aggression. En effet cet ambassadeur n"y a jamais ete autorise comme il l"a declare lui meme, et aussitot que j"en fus informe, je lui ai fait connaitre combien je le desapprouvais en lui donnant l"ordre de rester a son poste. Si Votre Majeste n"est pas intentionnee de verser le sang de nos peuples pour un malentendu de ce genre et qu"elle consente a retirer ses troupes du territoire russe, je regarderai ce qui s"est passe comme non avenu, et un accommodement entre nous sera possible. Dans le cas contraire, Votre Majeste, je me verrai force de repousser une attaque que rien n"a provoquee de ma part. Il depend encore de Votre Majeste d"eviter a l"humanite les calamites d"une nouvelle guerre.
Je suis, etc.
(signe) Alexandre.”
[“My lord brother! Yesterday it dawned on me that, despite the straightforwardness with which I observed my obligations towards Your Imperial Majesty, your troops crossed the Russian borders, and only now have I received a note from St. Petersburg, with which Count Lauriston informs me regarding this invasion, that Your Majesty considers yourself to be on hostile terms with me from the time Prince Kurakin demanded his passports. The reasons on which the Duke of Bassano based his refusal to issue these passports could never have led me to suppose that the act of my ambassador served as a reason for the attack. And in fact, he did not have a command from me to do this, as he himself announced; and as soon as I learned about this, I immediately expressed my displeasure to Prince Kurakin, ordering him to carry out the duties entrusted to him as before. If Your Majesty is not inclined to shed the blood of our subjects because of such a misunderstanding and if you agree to withdraw your troops from Russian possessions, then I will ignore everything that happened, and an agreement between us will be possible. Otherwise, I will be forced to repel an attack that was not provoked by anything on my part. Your Majesty, you still have the opportunity to save humanity from the scourge of a new war.
(signed) Alexander.” ]
On June 13, at two o'clock in the morning, the sovereign, calling Balashev to him and reading him his letter to Napoleon, ordered him to take this letter and personally hand it over to the French emperor. Sending Balashev away, the sovereign again repeated to him the words that he would not make peace until at least one armed enemy remained on Russian soil, and ordered that these words be conveyed to Napoleon without fail. The Emperor did not write these words in the letter, because he felt with his tact that these words were inconvenient to convey at the moment when the last attempt at reconciliation was being made; but he certainly ordered Balashev to hand them over to Napoleon personally.
Having left on the night of June 13th to 14th, Balashev, accompanied by a trumpeter and two Cossacks, arrived at dawn in the village of Rykonty, at the French outposts on this side of the Neman. He was stopped by French cavalry sentries.
A French hussar non-commissioned officer, in a crimson uniform and a shaggy hat, shouted at Balashev as he approached, ordering him to stop. Balashev did not stop immediately, but continued to walk along the road.
The non-commissioned officer, frowning and muttering some kind of curse, advanced with the chest of his horse towards Balashev, took up his saber and rudely shouted at the Russian general, asking him: is he deaf, that he does not hear what is being said to him. Balashev identified himself. The non-commissioned officer sent the soldier to the officer.
Not paying attention to Balashev, the non-commissioned officer began to talk with his comrades about his regimental affairs and did not look at the Russian general.
It was unusually strange for Balashev, after being close to the highest power and might, after a conversation three hours ago with the sovereign and generally accustomed to honors from his service, to see here, on Russian soil, this hostile and, most importantly, disrespectful attitude towards himself with brute force.
The sun was just beginning to rise from behind the clouds; the air was fresh and dewy. On the way, the herd was driven out of the village. In the fields, one by one, like bubbles in water, the larks burst into life with a hooting sound.
Balashev looked around him, waiting for the arrival of an officer from the village. The Russian Cossacks, the trumpeter, and the French hussars silently looked at each other from time to time.
A French hussar colonel, apparently just out of bed, rode out of the village on a beautiful, well-fed gray horse, accompanied by two hussars. The officer, the soldiers and their horses wore an air of contentment and panache.
This was the first time of the campaign, when the troops were still in good order, almost equal to the inspection, peaceful activity, only with a touch of smart belligerence in clothing and with a moral connotation of that fun and enterprise that always accompany the beginning of campaigns.
The French colonel had difficulty holding back a yawn, but was polite and, apparently, understood the full significance of Balashev. He led him past his soldiers by the chain and said that his desire to be presented to the emperor would probably be fulfilled immediately, since the imperial apartment, as far as he knew, was not far away.
They drove through the village of Rykonty, past French hussar hitching posts, sentries and soldiers saluting their colonel and curiously examining the Russian uniform, and drove out to the other side of the village. According to the colonel, the division chief was two kilometers away, who would receive Balashev and see him off to his destination.
The sun had already risen and shone cheerfully on the bright greenery.
They had just left the tavern on the mountain when a group of horsemen appeared from under the mountain to meet them, in front of which, on a black horse with harness shining in the sun, rode a tall man in a hat with feathers and black hair curled to the shoulders, in a red robe and with with long legs stuck out forward, like the French ride. This man galloped towards Balashev, his feathers, stones and gold braid shining and fluttering in the bright June sun.
Balashev was already two horses away from the horseman galloping towards him with a solemnly theatrical face in bracelets, feathers, necklaces and gold, when Yulner, the French colonel, respectfully whispered: “Le roi de Naples.” [King of Naples.] Indeed, it was Murat, now called the King of Naples. Although it was completely incomprehensible why he was the Neapolitan king, he was called that, and he himself was convinced of this and therefore had a more solemn and important appearance than before. He was so sure that he was really the Neapolitan king that, on the eve of his departure from Naples, while he was walking with his wife through the streets of Naples, several Italians shouted to him: “Viva il re!” [Long live the king! (Italian) ] he turned to his wife with a sad smile and said: “Les malheureux, ils ne savent pas que je les quitte demain! [Unhappy people, they don’t know that I’m leaving them tomorrow!]
But despite the fact that he firmly believed that he was the Neapolitan king, and that he regretted the grief of his subjects abandoned by him, recently, after he was ordered to enter the service again, and especially after his meeting with Napoleon in Danzig, when the august brother-in-law told him: “Je vous ai fait Roi pour regner a maniere, mais pas a la votre,” [I made you king in order to reign not in his own way, but in mine.] - he began cheerfully for a task familiar to him and, like a well-fed, but not fat, horse fit for service, sensing himself in the harness, began to play in the shafts and, having discharged himself as colorfully and expensively as possible, cheerful and contented, galloped, not knowing where or why, along the roads Poland.